Texas Immunization Registry (ImmTrac2)
DSHS courses are now available at the DSHS TX TRAIN platform.
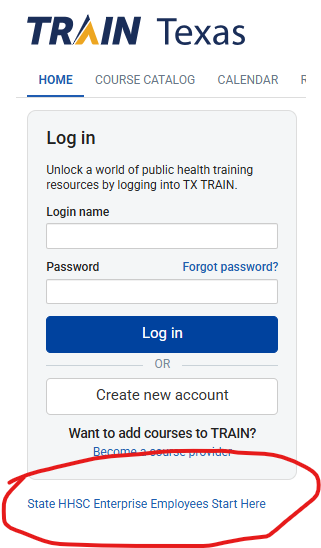
You will be asked to login (and create a profile if you do not have one). DSHS and HHSC employees should begin at the link indicated (circled below).
You can then search for your course by title.
https://www.train.org/texas/welcome